Heat Capacity of Ideal Gases
In statistical thermodynamics [175,138],
it is derived that each molecular degree of freedom contributes ![]() to the molar heat capacity of an ideal gas, where again
to the molar heat capacity of an ideal gas, where again ![]() is the
ideal gas constant.
is the
ideal gas constant.
An ideal monatomic gas molecule (negligible spin) has only
three degrees of freedom: its kinetic energy in the three spatial
dimensions. Therefore,
![]() . This means we expect
. This means we expect
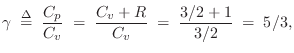
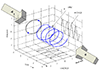
For an ideal diatomic gas molecule such as air, which can be pictured as a ``bar bell'' configuration of two rubber balls, two additional degrees of freedom are added, both associated with spinning the molecule about an axis orthogonal to the line connecting the atoms, and piercing its center of mass. There are two such axes. Spinning about the connecting axis is neglected because the moment of inertia is so much smaller in that case. Thus, for diatomic gases such as dry air, we expect
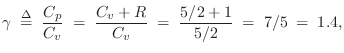
Next Section:
Speed of Sound in Air
Previous Section:
Adiabatic Gas Constant